yan McMullen had never heard of the USC Dornsife College of Letters, Arts and Sciences when he started casting about for a graduate chemistry program. But on the recommendation of one of his professors, he sent an email to the College's Professor of Chemistry Stephen Bradforth proposing an experiment to tease out what makes a metal really a metal.
The proposal would not only turn into his Ph.D. thesis but a major scientific breakthrough.
McMullen's proposal was not an easy sell. The experiment would be expensive and possibly dangerous.
The academics McMullen contacted at other U.S. research universities told him they had funding for their own research, but not for his. But Bradforth had a different response.
"He said, 'I don't have funding for your idea but if you come over here we can write a funding proposal together,'" said McMullen, who at the time was finishing up his undergraduate studies at the University of Bristol in the United Kingdom.
Bradforth not only helped McMullen secure funding, prioritizing it for National Science Foundation support over continuing other projects, but he also cobbled together an international team of scientists and arranged his sabbatical to oversee and participate in the main experiments. He also became McMullen's Ph.D. adviser.
Bradforth reconfigured his lab to protect its scientists. The experiment required liquid ammonia, which can be mildly toxic, and alkaline metal, which can explode if it touches water.
"My lab looks different because of this," noted Bradforth, who is also divisional dean for natural sciences and mathematics.
The effort was well worth the outcome. The experiment uncovered findings that are "the sort of things that go in textbooks, or at least changes how textbooks are written," Bradforth said, noting the potentially historic importance of the work. It would also earn the coveted distinction of being the June 5 cover of Science magazine.
Going full metal
The project looked at a fundamental question: Which properties are inherent to a metal and which are incidental?
Intuition suggests that metals are dense, and while that bears true for some (think gold or lead), it fails to hold up for others. For example, lithium—commonly used in batteries—floats on water. Some metals are hard, such as titanium, yet others yield easily to pressure, including indium and aluminum. How about melting temperature? Platinum melts at more than 1,700 degrees Celsius (3,200 F), but mercury is a liquid well below zero.
Many other definitions of 'metal-hood' suffer similar contradictions, but only metals are able to conduct electricity. Conduction, unlike density or hardness, is an inherent property of all metals.
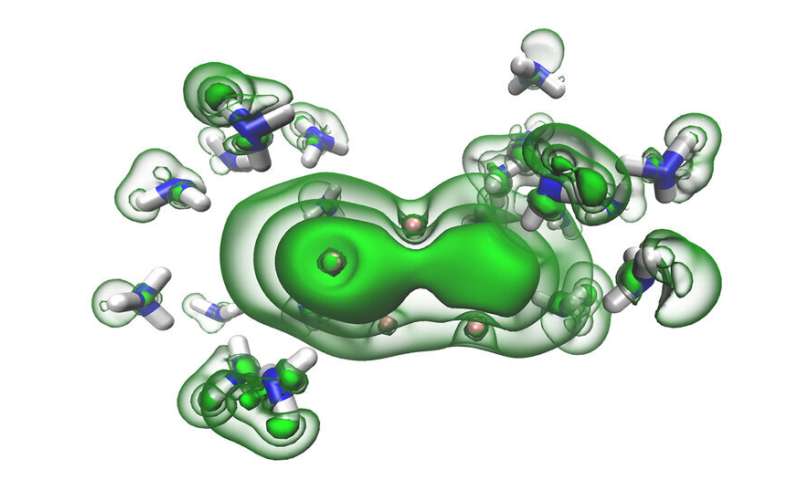
Seeking to further understand the intrinsic properties of metals, Bradforth, McMullen and their colleagues used a trick first noted by chemist Sir Humphry Davy in 1809. In essence, they made a metal from scratch.
The scientists cooled ammonia—normally a gas at room temperature—to minus 33 C to liquify it and then added, in separate experiments, the alkali metals lithium, sodium and potassium.
In these solutions, electrons from the alkali metal initially become trapped in the gaps between ammonia molecules. This creates what scientists call 'solvated electrons,' which are highly reactive but stabilized in the ammonia. These solutions have a characteristic blue color. But given enough solvated electrons, the whole liquid turns bronze and, in essence, becomes a metal while remaining liquid.
Solvated electrons have proven to be important to organic chemists. Through a reaction called the "Birch reduction," named after chemist Arthur Birch, they were key to synthesizing many important compounds and led to the manufacture of oral contraceptives in the 1950s.
Beaming in on electrons
The scientists next measured the amount of energy needed to bump the solvated electrons out of metallic ammonia using an extremely bright and focused X-ray beam based in Berlin.
In a first-ever experiment, they forced different concentrations of the metallic ammonia through a microjet, which created a stream about the width of a human hair that then passed through a hair-thin X-ray beam.
The results showed that, at low concentrations, solvated electrons were more easily dislodged from the solution by the interaction with the X-rays, giving a simple energy pattern. At higher concentrations, though, the energy pattern suddenly developed a sharp band edge, indicating the solution was behaving as a metal would.
While the practical implications of the outcome need further research, the experiment does open a new window for chemists to synthesize important organic compounds. Just as the Birch reduction led to oral contraceptives, so, too, could this experiment lead to new compounds for use in an untold number of ways.
Jersey Boy
McMullen, a native of Jersey (the European original, not the state neighboring New York), plans to return to his lab at USC Dornsife within a few weeks. But he hasn't let the COVID-19 pandemic slow him down. Always curious about how electronics work, he has been carrying out experiments—safely, of course—from his apartment in Long Beach, California, using components he purchased on e-Bay.
After completing his Ph.D., McMullen, the first in his family to attend college, plans to pursue a postdoctoral fellowship, though he's not sure where or what he will focus on. He does know, however, that he wants to remain in academia. Wherever he lands, it is almost certain that the world of chemistry will hear from him again.
"I like to do the exotic things."
Tillmann Buttersack et al. Photoelectron spectra of alkali metal–ammonia microjets: From blue electrolyte to bronze metal,
Science(2020).
DOI: 10.1126/science.aaz7607Citation: An experiment suggested by a Ph.D. student may rewrite chemistry textbooks (2020, June 23) retrieved 24 June 2020 from https://ift.tt/2BBQZJO
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
from Hacker News https://ift.tt/2BBQZJO
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.